science & platforms
The Frontier™ Platform
The Frontier™ Platform
Proprietary platform powered by chemoproteomics, covalent chemistry, and machine learning

The Frontier™ Platform
chemoproteomics
Chemoproteomics is an approach to understand the interaction between small molecules and proteins in cellular contexts.
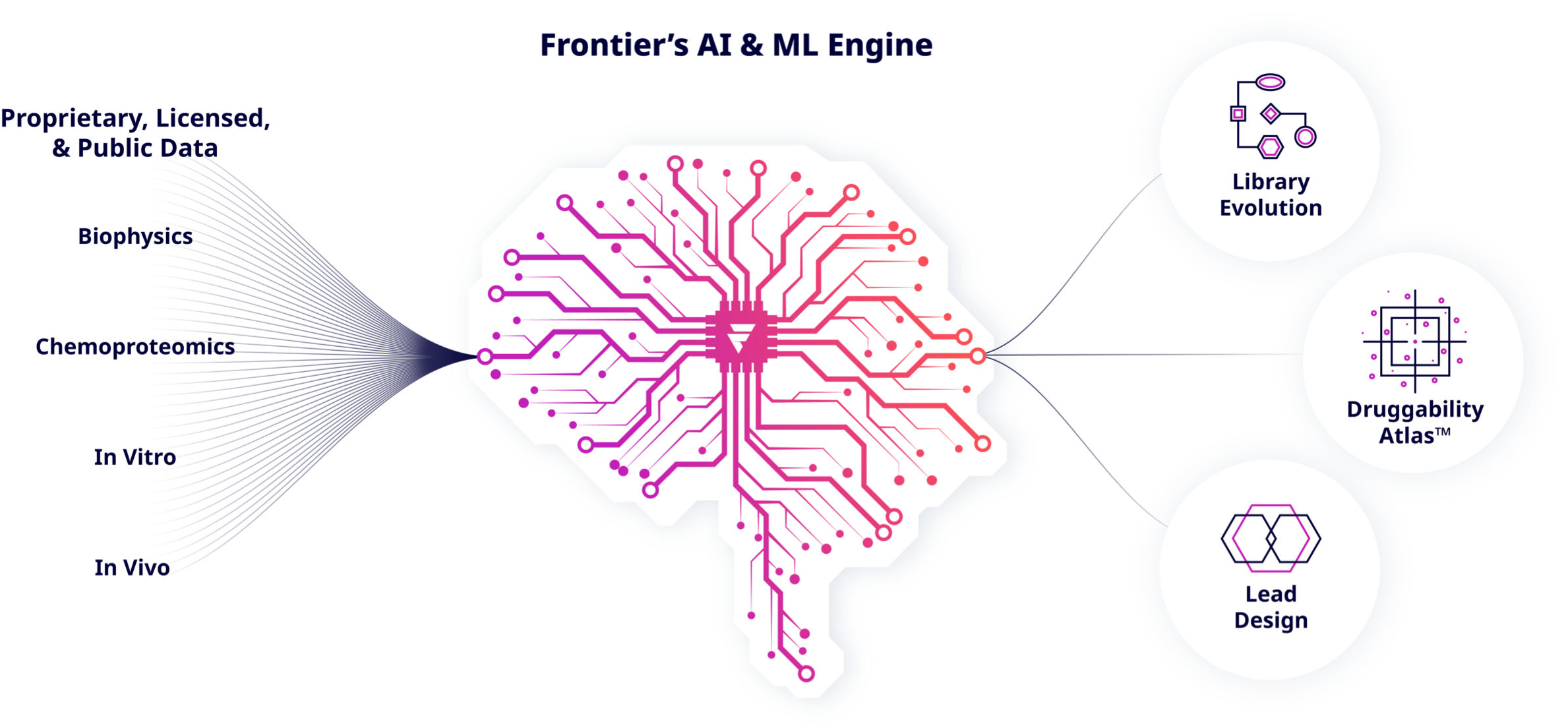
Frontier uses chemoproteomics paired with machine learning algorithms to discover binding pockets (or druggable hotspots) within proteins involved in critical disease pathways, then targets them pharmacologically. Frontier’s proprietary drug discovery capabilities have been built to accelerate the discovery and development of precision medicines. Our platform integrates a set of leading proteomic capabilities, generating billions of data points stored within our proprietary hotspot database, with a data-driven and evolving covalent fragment library. Insights from proteome screening in disease relevant systems are combined with those from cutting edge mass spectrometry, machine-learning algorithms, modeling, simulation, and structural information, alongside innovative medicinal chemistry.
Understanding Frontier’s Technology
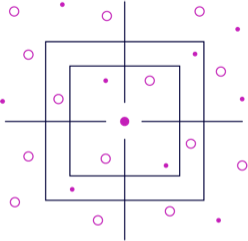
Druggability AtlasTM
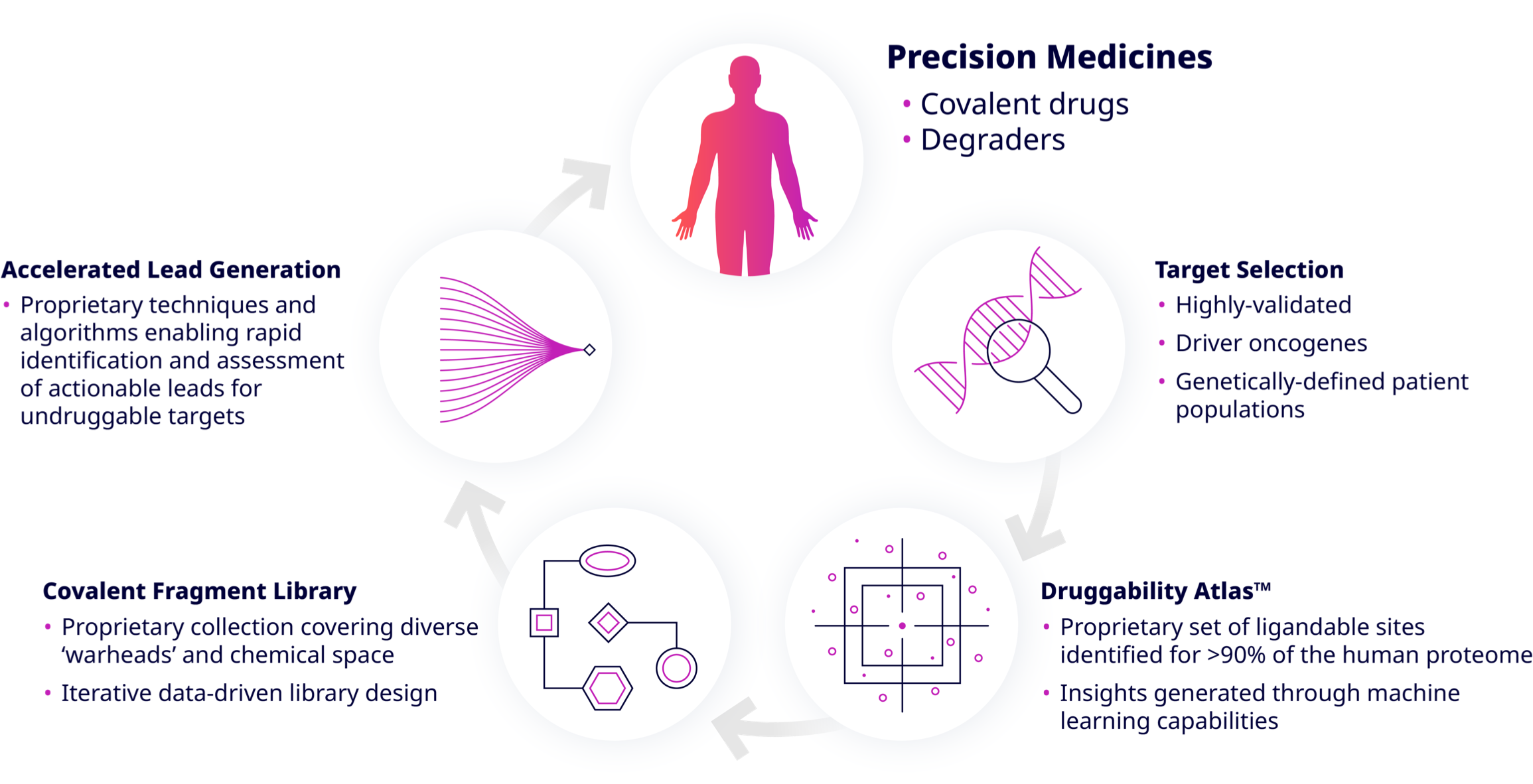
Frontier’s technology interrogates the proteome and delivers an atlas of binding sites on targets of interest, allowing for the development of small molecules against practically any protein. Because proteins behave differently in different cellular environments, our data is collected across disease systems that are carefully selected to produce rich and relevant datasets.
The Druggability AtlasTM serves as the starting point for understanding the druggability of a potential therapeutic target. Frontier has identified >150,000 hotspots enabling access to > 90% of the human proteome for drug discovery.
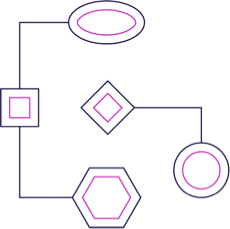
Covalent Fragment Library
Frontier has established a proprietary covalent fragment library using machine learning models that leverage Frontier’s databases. Small molecule fragments are screened to identify those that can bind to priority hotspots. Fragments are less complex than molecules in typical high-throughput screening libraries and therefore have a higher chance of binding to unique pockets on proteins including undruggable proteins.
Accelerated Lead Generation
Frontier’s covalent fragment library is screened against multiple targets using high throughput biophysical, biochemical, and biological technologies. In particular, Frontier’s high-throughput protein mass spectrometry platform can run thousands of intact protein samples per day. Fragments that bind selectively are then further studied using high resolution mass spectrometry to determine binding kinetics, the site of modification and proteome-wide selectivity. Once validated, specific fragment hits are rapidly advanced using medicinal chemistry, as well as computer-assisted and structure-based drug design.
Our platform and strategy allow us to rapidly de-risk a program, converting a historically undruggable target into a tractable project that can be accelerated through lead optimization and onward to the clinic.
Machine Learning / Artificial Intelligence
Frontier has developed machine learning algorithms that leverage our unique hotspot data to classify, for a given protein, the relative reactivities and the binding potential of specific regions. This enables not only the prioritization of particular targets directly from our database, but also the design of effective screening strategies.

Artificial intelligence approaches are also applied to generate and curate Frontier’s covalent fragment library.
Frontier has developed and deployed algorithms that determine the desired chemical property space and reactivity profiles based on the latest data. Such data-driven insights enable us to focus on the most promising areas of chemical space.
These machine learning applications enable Frontier to generate an expanding library of high quality and diverse compounds against key targets and their prioritized hotspots. This opens novel opportunities to enable the development of innovative precision-based medicines.
Frontier's Machine Learning/AI Engine